CDK regulation of the meiotic nuclear biology
A key feature of meiosis, the cellular program that generates gametes, is the recombination between homologous chromosomes (maternal and paternal) that, in addition to generate genetic diversity, facilitates accurate chromosome segregation. Defects in chromosome segregation during meiosis cause miscarriages, infertility and genetic diseases, revealing the importance of understanding the meiotic recombination process. Our laboratory is interested in how this particular recombination is regulated. To this aim we are using the fission yeast Schizosaccharomyces pombe, since this organism is a proven model system for different eukaryotic conserved processes.
Recombination initiates with programmed DNA double-strand breaks (DSBs) in the context of linear elements (LinEs) -dynamic chromosome structures similar to the synaptonemal complex of other eukaryotes- that serve as platforms where the pre-recombination complexes, which eventually will generate the breaks in the DNA, are loaded. A strict regulation of DSB formation and repair during meiosis is critical to maintain the genome integrity during this physiological situation, in which self-inflicted DNA damage is used as a source of genetic recombination. Thus, DNA break formation and repair have to be coordinated with meiotic progression to ensure proper chromosome distribution and faithful genome transmission into meiotic products.
Our previous work identified several components of LinEs and pre-recombination complexes, as well as functional relationships among them, and key protein residues required for LinE organization/localization and DSB formation. More recently, we have found that CDK (cyclin-dependent kinase) activity, which controls meiotic progression, also regulates DSB formation in fission yeast. Maturation of LinEs seems to be an important point of CDK regulation, modulating the binding to chromatin of one of their structural components. In addition, apart from the prominent role in DSB formation of the meiosis-specific Crs1 cyclin, we have recently identified additional functions. This cyclin is required for meiotic S-phase progression, a canonical role, and to maintain the architecture of the meiotic chromosomes. Crs1 localizes at the SPB (Spindle Pole Body; centrosome equivalent in yeast) and is required to stabilize the cluster of telomeres at this location (bouquet configuration), as well as for normal SPB motion. The maintenance of the meiotic nuclear architecture as well as the nuclear movements are essential for an efficient recombination, and therefore for the production of viable gametes. Thus, the meiosis-specific Crs1 cyclin seems to be a multitask cyclin with roles in several key aspects of meiosis.
Furthermore, our work indicates that CDK activity might also control downstream events balancing the repair pathways after DSB formation. Hence, CDK activity in meiosis may regulate different steps of the recombination process. We are currently exploring new points of this regulation and found that CDK phosphorylates Sfr1 and restricts the function of the Swi5-Sfr1 accessory factor to dismantle Rad51-mediated homolog invasions prior to chromosome segregation. In doing so, CDK guarantees timely resolution of recombination intermediates and successful chromosome distribution into the gametes.
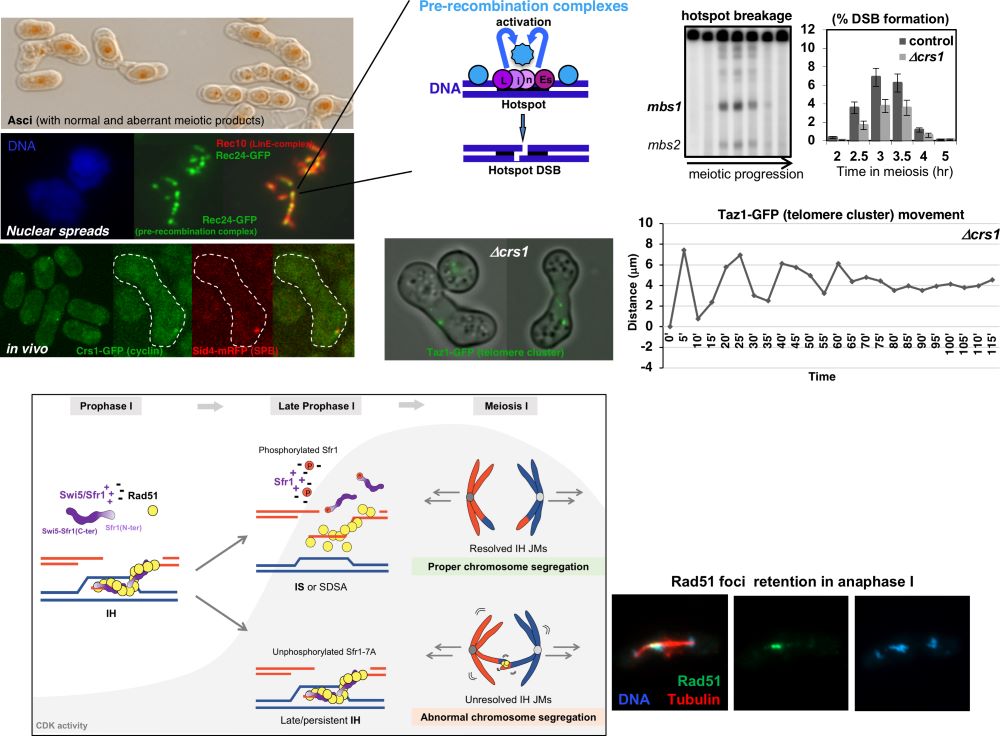
Figure.Consequences of abnormal meiotic recombination in the formation of sexual products (spores); cytological localization of proteins required for the initiation of meiotic recombination; physical analysis of DSB formation, cytological organization and dynamics of the telomere-cluster in crs1 (cyclin) mutants; Sfr1 phospho-inhibition by CDK and Rad51 foci retention when missregulated.


Miembros del grupo
| Cristina Martín Castellanos | Científico Titular (CSIC) ORCID: 0000-0002-1303-2517 ResearcherID: F-4502-2017 |
|---|---|
| Josué I. Ortega Lucero | Estudiante de Máster |
Contacto
| Cristina Martín Castellanos |
cmartin@usal.es 923294924 Laboratorio 2.11 |
|---|
Publicaciones recientes
| Palacios-Blanco I, Gómez L, Bort M, Mayerová N, Bágeľová Poláková S, Martín-Castellanos C (2024)
CDK phosphorylation of Sfr1 downregulates Rad51 function in late-meiotic homolog invasions. EMBO J. Aug 22. Doi: 10.1038/s44318-024-00205-2 |
| Palacios-Blanco, I and Martín-Castellanos, C (2022)
Cyclins and CDKs in the regulation of meiosis-specific events Frontiers in Cell and Developmental Biology 10:1069064 Doi: 10.3389/fcell.2022.1069064 |
| Bustamante-Jaramillo LF, Ramos C, Martín-Castellanos C (2021)
The Meiosis-Specific Crs1 Cyclin Is Required for Efficient S-Phase Progression and Stable Nuclear Architecture. Int J Mol Sci. 22(11): 5483 Doi: 10.3390/ijms22115483. |
| Morafraile EC, Bugallo A, Carreira R, Fernández M, Martín-Castellanos C, Blanco MG, Segurado M (2020)
Exo1 phosphorylation inhibits Exonuclease activity and rescues fork collapse in rad53 mutants independently of the 14-3-3 proteins. Nucleic Acids Research gkaa054 Doi: 10.1038/s44318-024-00205-2 |
| Bustamante-Jaramillo LF, Ramos C, Alonso L, Sesmero A, Segurado M, Martín-Castellanos C (2019)
CDK contribution to DSB formation and recombination in fission yeast meiosis. PLoS Genetics. 15(1):e1007876 Doi: 10.1371/journal.pgen.1007876 |
| Ma L, Fowler KR, Martín-Castellanos C, Smith GR (2017)
Functional organization of protein determinants of meiotic DNA break hotspots. Scientific Reports 7:1393 Doi: 10.1038/s41598-017-00742-3 |
| Martín-Castellanos C, Fowler KR and Smith GR (2013)
Making chromosomes hot for breakage. Cell Cycle 12: 1327-1328 Doi: 10.4161/cc.24576 |
| Fowler KR, Gutiérrez-Velasco S, Martín-Castellanos C and Smith GR (2013)
Protein determinants of meiotic DNA break hotspots. Mol. Cell 49: 983-996 Doi: 10.1016/j.molcel.2013.01.008 |
Proyectos de investigación
| Junta de Castilla y León: CSI010P23 |
| Junta de Castilla y León: CSI259P20 |
| MICIU (PGC2018-101908-B-IOO/BMC) |
| MINECO (BFU2013-45182-P) |
| Unidad de Investigación Consolidada de Castilla y León: UIC 028 |







